Project P5 | Katia Cosentino
Plasticity of GSDM pores for the control of pyroptotic cell death
We analyze the role of the membrane environment on regulatory mechanisms of Gasdermin recruitment and pore formation at the plasma membrane in pyroptosis, an inflammatory form of regulated cell death.
© Katia Cosentino
Project Summary
Juniorprof. Dr. Katia Cosentino
Osnabrück University
School of Biology/Chemistry
Research Group Molecular Cell Biophysics

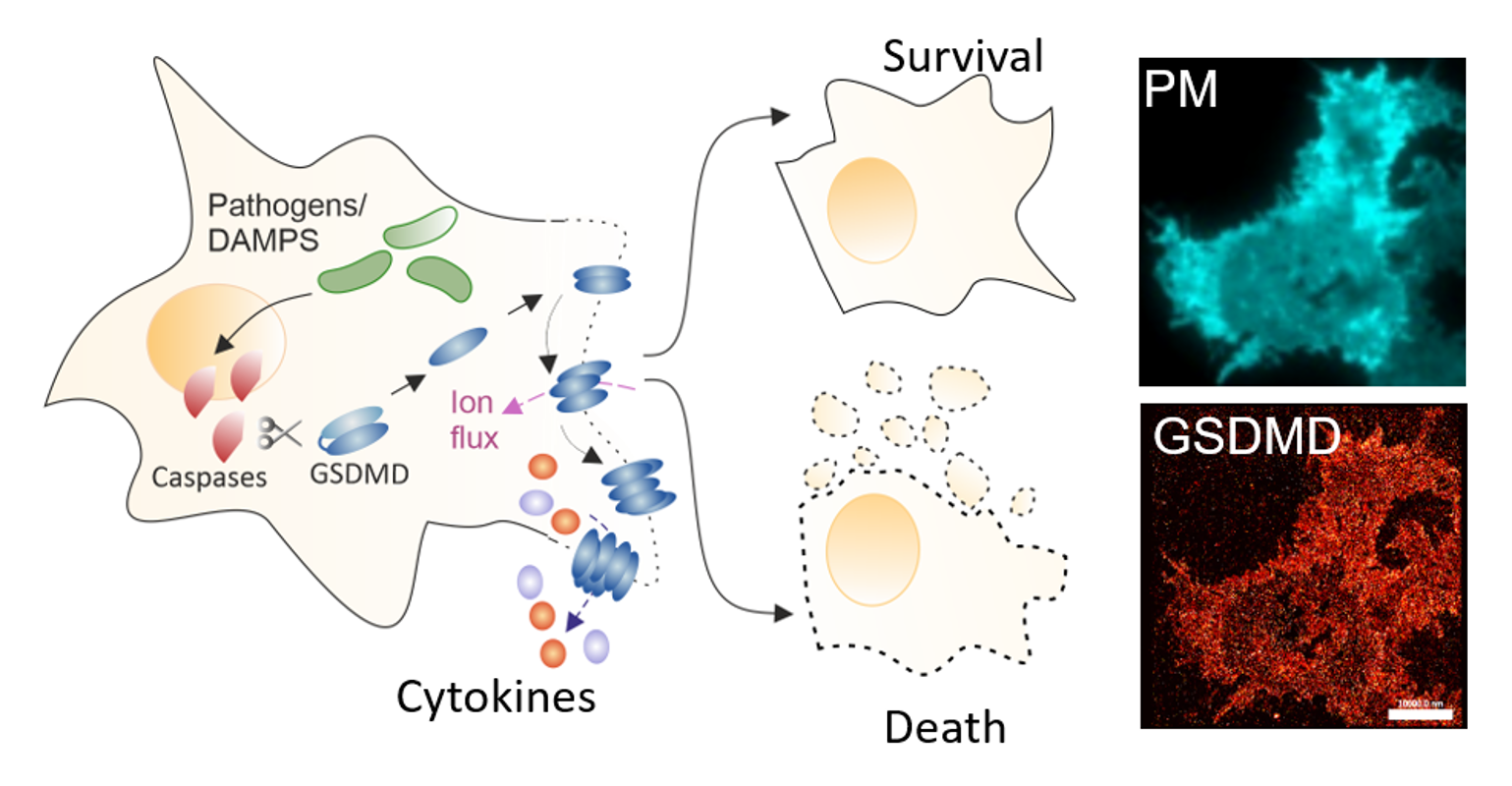
Members of the Gasdermin (GSDM) protein family are key executors of the plasma membrane (PM) permeabilization in pyroptosis, a highly inflammatory form of regulated cell death. This process is key in antimicrobial and inflammatory host responses but detrimental in severe inflammation-related diseases.
GSDM pores at the PM allow the extracellular release of cytokines and eventually lead to complete cell lysis with strong local inflammation. Recent observations, however, have shown that GSDM pores can allow the secretion of cytokines in the absence of cell death, thus reducing local inflammation in favor of adaptive immunity. What controls such functional plasticity of the pores and the resulting final cell fate (life vs. death) is entirely unclear but an understanding of this process would be essential to modulate inflammatory responses in medical settings.
Our group has previously dissected structural and mechanistic aspects of pore assembly by the GSDM family member GSDMD at the PM of pyroptotic cells by high-resolution single-molecule fluorescence microscopy. Our observations highlight a direct involvement of the lipid environment in the functional plasticity of GSDMD pores and suggest that functional plasticity is linked to pore structural plasticity. In this project, we aim to uncover how the membrane environment contributes to the structural plasticity of the pores to control cell fate.
Project-related publications
Cosentino, K., Hertlein, V., Jenner, A., Dellmann, T., Gojkovic, M., Peña-Blanco, A., Dadsena, S., Wajngarten, N., Danial, J.S.H., Thevathasan, J.V., et al. (2022). The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol Cell 82, 933-949.e939.
Danial, J.S.H., Quintana, Y., Ros, U., Shalaby, R., Margheritis, E.G., Chumpen Ramirez, S., Ungermann, C., Garcia-Saez, A.J., Cosentino, K. (2022). Systematic Assessment of the Accuracy of Subunit Counting in Biomolecular Complexes Using Automated Single-Molecule Brightness Analysis. J Phys Chem Letters 13, 822-829.
Cosentino, K., Hermann, E., von Kügelgen, N., Unsay, J.D., Ros, U., García-Sáez, A.J. (2021). Force Mapping Study of Actinoporin Effect in Membranes Presenting Phase Domains. Toxins 13, 669.
Voskoboynikova, N., Margheritis, E.G., Kodde, F., Rademacher, M., Schowe, M., Budke-Gieseking, A., Psathaki, O.-E., Steinhoff, H.-J., Cosentino, K. (2021). Evaluation of DIBMA nanoparticles of variable size and anionic lipid content as tools for the structural and functional study of membrane proteins. BBA - Biomembranes 1863, 183588.
Jenner, A., Shalaby, R., Cosentino, K. (2020). Chapter Three - Quantitative single-molecule imaging of protein assembly in membranes. In Advances in Biomembranes and Lipid Self-Assembly, pp. 81-128. (Review)
Kuwana, T., King, L.E., Cosentino, K., Suess, J., Garcia-Saez, A.J., Gilmore, A.P., and Newmeyer, D.D. (2020). Mitochondrial residence of the apoptosis inducer BAX is more important than BAX oligomerization in promoting membrane permeabilization. J Biol Chem 295, 1623-1636.
Cosentino, K., García-Sáez, A.J. (2017). Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol 27, 266-275. (Review)
Unsay, J.D., Cosentino, K., Sporbeck, K., García-Sáez, A.J. (2017). Pro-apoptotic cBid and Bax exhib-it distinct membrane remodeling activities: An AFM study. BBA - Biomembranes 1859, 17-27.
Salvador-Gallego, R., Mund, M., Cosentino, K., Schneider, J., Unsay, J., Schraermeyer, U., Engelhardt, J., Ries, J., García‐Sáez, A.J. (2016). Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J 35, 389-401.
Subburaj, Y., Cosentino, K., Axmann, M., Pedrueza-Villalmanzo, E., Hermann, E., Bleicken, S., Spatz, J., García-Sáez, A.J. (2015). Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat Commun 6, 8042.