Project P1 | Caroline Barisch
Remodelling and exploitation of the host lipid trafficking machinery by pathogenic mycobacteria
We exploit Dictyostelium infected with mycobacteria to investigate how pathogens reprogram metabolic lipid flows and impact the functional plasticity of the mycobacteria-containing vacuole membrane composition.
© Caroline Barisch
Project Summary
Dr. Caroline Barisch
Osnabrück University
School of Biology/Chemistry
Research Group Molecular Infection Biology

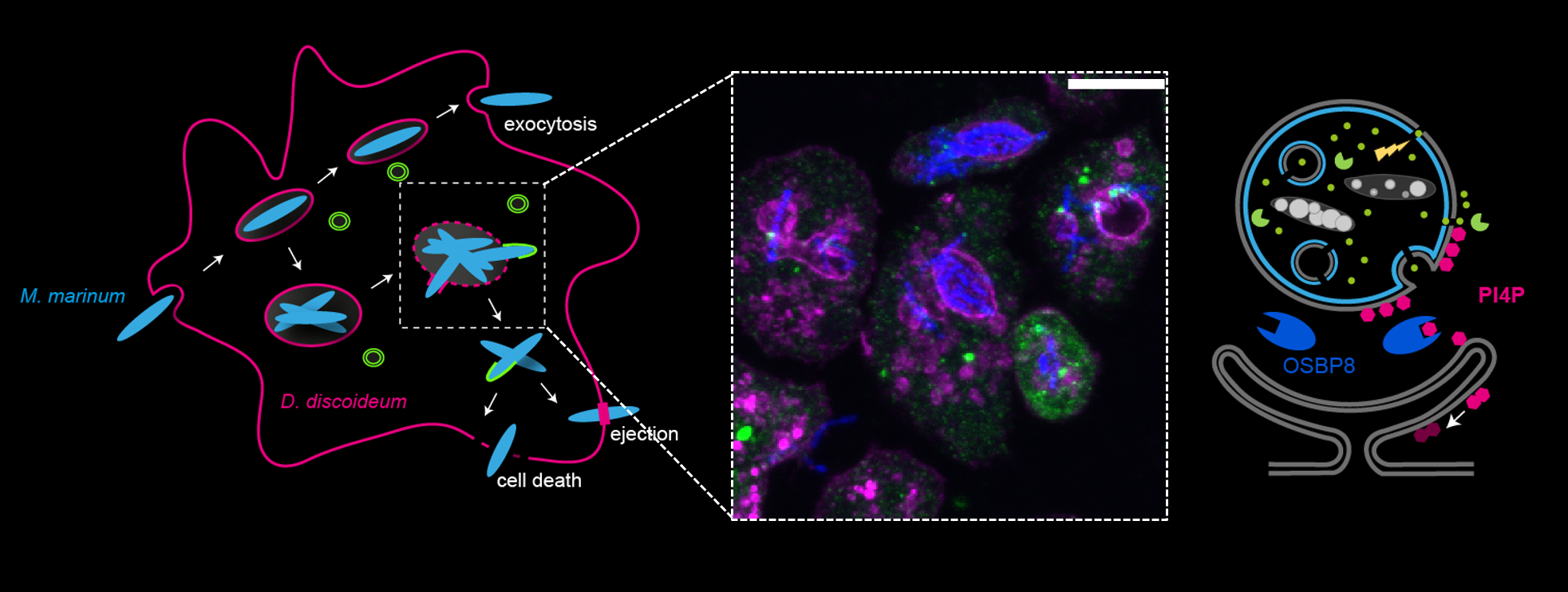
Bacterial pathogens are masters in remodelling the lipid metabolic network of their host to create an optimal environment for their proliferation. A recurrent strategy is the induction of membrane contact sites (MCS) between biogenic host organelles and bacteria-containing vacuoles to create focal points for lipid exchange.
Using the Dictyostelium discoideum/ Mycobacterium marinum infection system as a model for tuberculosis (Tb), we found that pathogenic mycobacteria selectively recruit oxysterol binding protein (OSBP) 8 to the mycobacteria-containing vacuole (MCV). Interestingly, non-pathogenic bacteria that lack the type VII secretion system ESX-1 fail to mobilize OSBP8 to the MCV, suggesting that specific mycobacterial effector proteins participate in vacuolar recruitment of OSBP8.
Our findings offer a fresh starting point to unravel how pathogenic mycobacteria alter the lipid and protein composition of the MCV membrane to gain access to host sterols. Consequently, functional plasticity is here defined as the remodelling of the lipid and protein composition of the MCV membrane upon infection to induce the formation of MCS and to recruit lipid transfer proteins (LTPs). Precise knowledge of the underlying molecular principles and their relevance for phagosome escape, replication and metabolic activity of mycobacteria is of key interest to understand the mechanisms of Mycobacterium tuberculosis (Mtb) pathogenicity, the causative agent of Tb.
In this project, we will exploit Dictyostelium infected with mycobacteria that are proficient or defective in the secretion of particular bacterial effectors to investigate how these pathogens reprogram metabolic lipid flows and consequently impact on the functional plasticity of the MCV membrane composition.
Project-related Publications
Foulon, M., Listian, S.A., Soldati, T., Barisch, C.(2022) Chapter 7. Conserved mechanisms drive host lipid access, import and utilisation in Mycobacterium tuberculosis and M. marinum. In Biology of Mycobacterial Lipids, Z. Fatima, and S. Canaan, eds. (Elsevier).
Hanna, N., Koliwer-Brandl, H., Lefrançois, L.H., Kalinina, V., Cardenal-Muñoz, E., Appiah, J., Leuba, F., Hilbi, H., Soldati, T., Barisch, C. (2021) Zinc intoxication of Mycobacterium marinum during Dictyostelium discoideum infection is counteracted by induction of the pathogen Zn2+ exporter CtpC. mBio, e01313-20.
Niekamp, K., Guzman, G., Leier, H., Rashidfarrokhi, A., Richina, V., Pott, A., Barisch, C., Holthuis J, Tafesse, F. (2021) Sphingomyelin biosynthesis is essential for phagocytic signaling during Mycobacterium tuberculosis host cell entry. mBio, e03141-20.
Luscher, A., Fröhlich, F., Barisch, C., Littlewood, C., Metcalfe, J., Leuba, F., Palma, A., Pirruccello, M., Cesareni, G., Stagi, M., Walther, T.C., Soldati, T., De Camilli, P., Swan, L.E. (2019) Lowe Syndrome-linked endocytic adaptors direct membrane cycling kinetics with OCRL in Dictyostelium discoideum. Mol Biol Cell 30, 2268-2282.
Koliwer-Brandl, H., Knobloch, P., Barisch, C., Welin, A., Hanna, N., Soldati, T., Hilbi, H. (2019) Distinct Mycobacterium marinum phosphatases determine pathogen vacuole phosphoinositide pattern, phagosome maturation, and escape to the cytosol. Cell Microbiol 21, e13008.
López-Jiménez, A.T., Cardenal-Muñoz, E., Leuba, F., Gerstenmaier, L., Barisch, C., Hagedorn M., King, J.S., Soldati, T. (2018) The ESCRT and autophagy machineries cooperate to repair ESX-1-dependent damage at the Mycobacterium-containing vacuole but have opposite impact on containing the infection. PLoS Pathog 31, e1007501.
Barisch C., Kalinina, V., Lefrancois, L., Appiah, J., Lopez-Jimenez, A.T., Soldati, T. (2018) Localization of all four ZnT zinc transporters in Dictyostelium and impact of ZntA and B knockout on bacteria killing. J Cell Sci 30, 131.
Barisch, C., Soldati, T. (2017) Breaking fat! How mycobacteria and other intracellular pathogens manipulate host lipid droplets. Biochimie 141, 54-61. (Review)
Barisch, C., Soldati, T. (2017) Mycobacterium marinum degrades both triacylglycerols and phospholipids from its Dictyostelium host to synthesise its own triacylglycerols and generate lipid inclusions. PLoS Pathog 13, e1006095.
Barisch, C., Paschke, P., Hagedorn, M., Maniak, M., Soldati, T. (2015) Lipid droplet dynamics at early stages of Mycobacterium marinum infection in Dictyostelium. Cell Microbiol 17, 1332-49.








