Project P11 | Arne Möller
Membrane determinants for ABC transporter plasticity
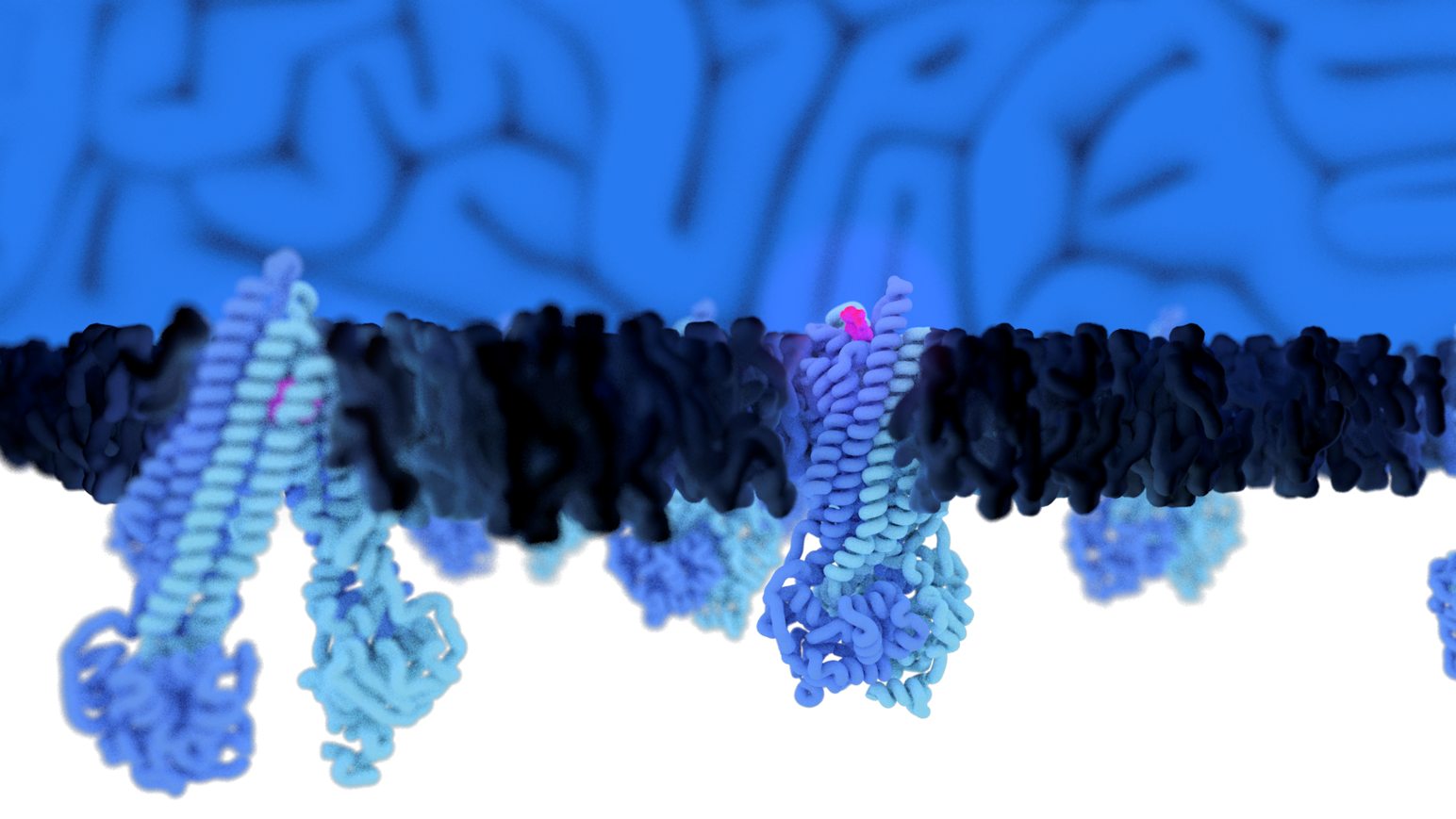
Using cryo-electron microscopy, we analyze the structure, dynamics and function of plasma membrane ABC transporters to understand their regulation by the membrane environment.
© Arne Möller
Project Summary
Prof. Dr. Arne Möller
Osnabrück University
School of Biology/Chemistry
Research Group Structural Biology

ATP binding cassette (ABC) transporters are a family of integral membrane proteins that actively translocate various substances across cellular membranes and are highly relevant targets for developing therapeutical drugs. Conformational dynamics and activity of ABC transporters are directly tied to the composition and local organization of the hydrophobic environment and lipid bilayer. As such, high-resolution structures of ABC transporters often display structured lipids and cholesterol at conserved positions on the transmembrane domains, indicating that they closely interact with their surrounding lipids and are immediately affected by the shape, geometry, and composition of the membrane.
How such membrane plasticity affects the conformational spectrum of ABC transporters remains, despite their fundamental importance in health and disease, entirely unclear. Consequently, our understanding of structural dynamics of ABC transporters is biased by the specific hydrophobic conditions, skewing the interpretation of the data.
Structural effects on membrane proteins elicited by lipid composition or lateral pressure are generally poorly understood; however fundamental for the development of therapeutics, where it is mandatory to comprehend the substrate binding and translocation mechanism. This project will focus on the connection between membrane plasticity and conformational plasticity of ABC transporters.
Project-related publications
Hofmann, S., Januliene, D., Mehdipour, A.R., Thomas, C., Stefan, E., Brüchert, S., Kuhn, B.T., Geertsma, E.R., Hummer, G., Tampé, R. Moeller, A. (2019). Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature 571, 580–583.
Blees, A., Januliene, D., Hofmann, T., Koller, N., Schmidt, C., Trowitzsch, S., Moeller, A., Tampé, R. (2017). Structure of the human MHC-I peptide-loading complex. Nature 551, 525–528.
Timcenko, M., Lyons, J.A., Januliene, D., Ulstrup, J.J., Dieudonné, T., Montigny, C., Ash, M.-R., Karlsen, J.L., Boesen, T., Kühlbrandt, W., Lenoir, G., Moeller, A., Nissen, P. (2019). Structure and autoregulation of a P4-ATPase lipid flippase. Nature 571, 366-370.
Shvarev, D., Januliene, D., Moeller, A. (2022). Frozen motion: how cryo-EM changes the way we look at ABC transporters. Trends Biochem Sci 47, 136-148. (Review)
Moeller, A., Lee, S.C., Tao, H., Speir, J.A., Chang, G., Urbatsch, I.L., Potter, C.S., Carragher, B., Zhang, Q.(2015). Distinct Conformational Spectrum of Homologous Multidrug ABC Transporters. Structure 23, 450-460.
Tao, H., Lee, S.C., Moeller, A., Roy, R.S., Siu, F.Y., Zimmermann, J., Stevens, R.C., Potter, C.S., Carragher, B., Zhang, Q. (2013). Engineered nanostructured β-sheet peptides protect membrane proteins. Nature Meth 10, 759-761.
Bhabha, G., Cheng, H.-C., Zhang, N., Moeller, A., Liao, M., Speir, J.A., Cheng, Y., Vale, R.D. (2014). Allosteric communication in the dynein motor domain. Cell 159, 857–868.
Leung, J.H., Schurig-Briccio, L.A., Yamaguchi, M., Moeller, A., Speir, J.A., Gennis, R.B., Stout, C.D. (2015). Division of labor in transhydrogenase by alternating proton translocation and hydride transfer. Science 379, 178-181.








