Project P6 | Florian Fröhlich
Plasticity of the serine palmitoyl transferase complex during adaption of sphingolipid metabolism
We determine the function and regulation of the key enzyme in sphingolipid metabolism, the serine palmitoyltransferase (SPT) enzyme complex to understand metabolic sphingolipid fluxes.
© Florian Fröhlich
Project Summary
Prof. Dr. Florian Fröhlich
Osnabrück University
School of Biology/Chemistry
Bioanalytical Chemistry

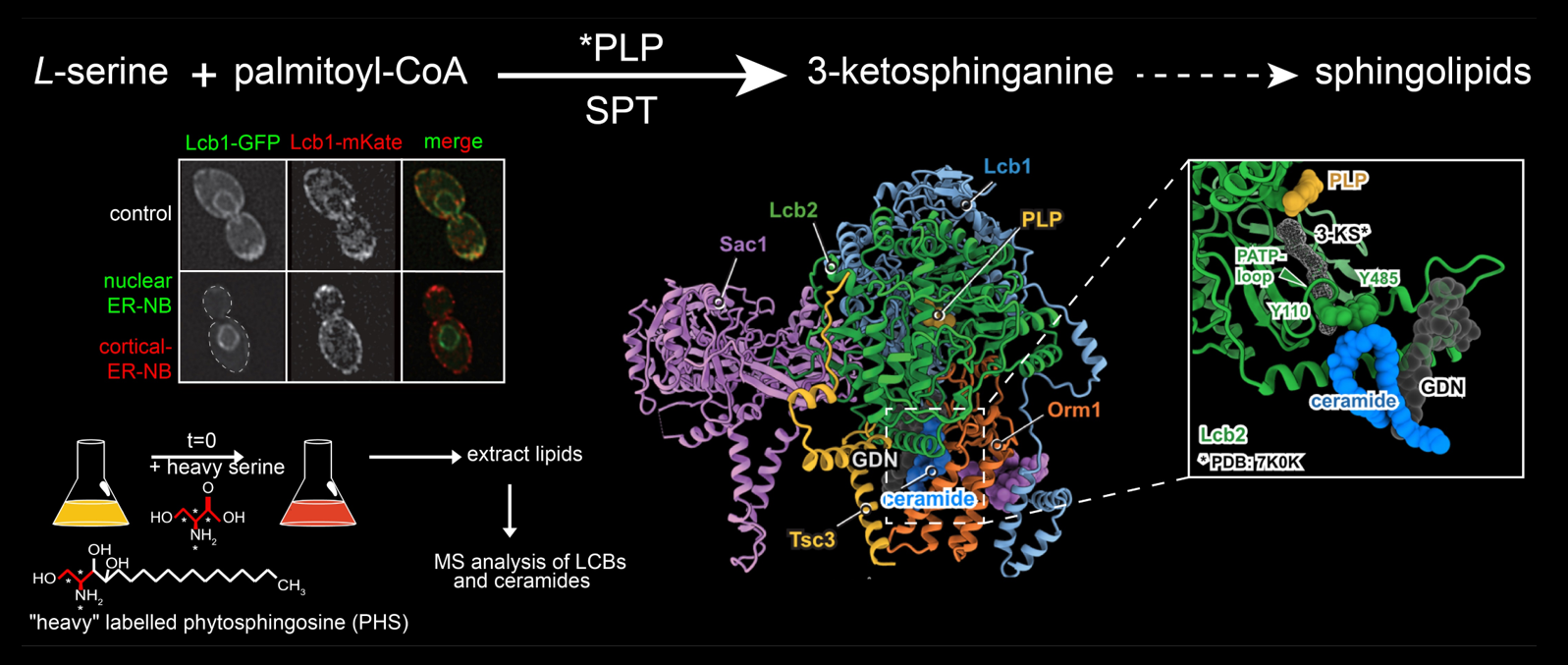
Sphingolipids (SLs) are one of the three major lipid classes in eukaryotic cells and are structural components of the plasma membrane. The rate limiting step in SL biosynthesis is catalysed by the serine palmitoyl-transferase (SPT).
In yeast, the SPT consists of the two catalytic subunits Lcb1 and Lcb2 and the regulatory subunit Tsc3. In addition, the SPT forms a complex with the negative regulators Orm1 and Orm2 and the phosphatidylinositol-4-phosphate (PI4P) phosphatase Sac1 (SPOTS complex). The Orm subunits are phosphorylated by two signalling pathways emerging either from the plasma membrane localized TOR complex 2 or the lysosomal/vacuolar TOR complex 1.
Regulation of the SPT is crucial to control organization of the plasma membrane and is key for the cells to adapt to changing environmental conditions. For example, SPT activity and thus SL biosynthesis is upregulated by one to two orders of magnitude upon heat stress. However, whether SPT regulation is spatially organized in cells and the precise regulatory function of all subunits of the SPOTS complex remain elusive. Here, we will analyse SPT regulation at the sub-cellular and the molecular level utilizing two different aims.
Project-related Publications
Bisinski, D.D., Gomes Castro, I., Mari, M., Walter, S., Fröhlich, F., Schuldiner, M., González Montoro, A. (2022) A novel component of multiple vacuolar contact sites is required for sphin-golipid homeostasis. J Cell Biol. In Press.
Körner, C., Fröhlich, F. (2022) Compartmentation and functions of sphingolipids. Curr Opin Cell Biol 74:104-111.
Eising, S., Esch, B.M., Wälte, M., Vargas Duarte, P., Walter, S., Ungermann, C., Bohnert, M., Fröhlich, F. (2022) A yeast lysosomal biogenesis map uncovers the cargo spectrum of lysosomal protein targeting pathways. J Cell Biol 222, e202107148.
Erdbrügger, P., Fröhlich F. (2020) The role of very long chain fatty acids in yeast physiology. Biol Chem 402, 25-38. (Review)
Esch, B.M., Limar, S., Bogdanowski, A., Gournas, C., Sundag, C., Walter, S., Kost, C., Heinisch, J.J., André, B., Fröhlich, F. (2020) Uptake of exogenous serine is important to maintain sphingolipid homeostasis in Saccharomyces cerevisiae PLoS Genet 16, e1008745.
Ponsford, A.H., Raimondi, A., Cocucci, E., Wycislo, S.A., Fröhlich, F., Swan, L.E., Stagi, M. (2020) Live imaging of intra-lysosome pH in cell lines and primary neuronal culture using a novel genetically encoded biosensor. Autophagy 17, 1500-1518.
Schmidt, O., Weyer, Y., Baumann, V., Widerin, M.A., Eising, S., Angelova, M., Schleiffer, A., Kremser, L., Lindner, H., Peter, M., Fröhlich, F., Teis, D. (2019) Post-ER organelle associated degradation of membrane proteins regulates sphingolipid metabolism. EMBO J 38, e101433.
Eising, S., Thiele, L., Fröhlich, F. (2019) A systematic approach to identify recycling endocytic cargo depending on the GARP complex. Elife 8, e42837.
Fröhlich, F., Olson, DK., Christiano, R., Farese, Jr. R.V., Walther, T.C. (2016) Proteomic and Phosphoproteomic Analyses of Yeast Reveal the Global Cellular Response to Sphingolipid Depletion. Proteomics 16, 2759-2763.
Fröhlich, F., Petit, C., Kory, N., Christiano, R., Hannibal-Bach, H.K., Graham, M., Liu, X., Ejsing, C.S., Farese, R.V. Jr., Walther, T.C. (2015) The GARP Complex Is Required for Cellular Sphingolipid Homeostasis. Elife 4, e08712.