Project P10 | Daniel Kümmel
Adaptation of a Rab/GEF module for initiation and maintenance of planar cell polarity and cilia
We investigate the regulation of organelle identity by a guanine nucleotide exchange factor (GEF) complex involved in planar cell polarity, termed Fuzzy-Inturned, and its substrate, Rab23 GTPase using a structure-function based approach.
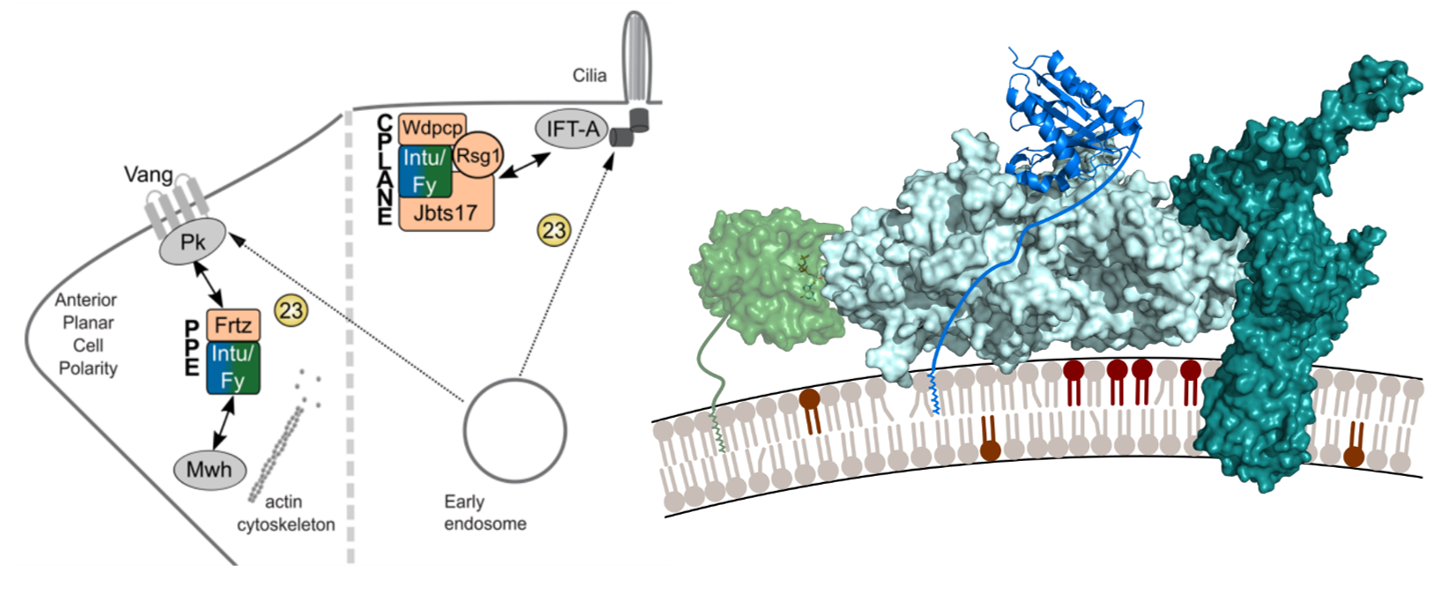
We are interested in the interplay of membrane and protein dynamics that control key developmental processes like callogenesis and planar cell polarity. In this context, we study Rab GTPases, which are required for maintenance of organelle identity and vesicular trafficking, and focus on Rab23 and its regulators. © Daniel Kümmel
Project Summary
Prof. Dr. Daniel Kümmel
University of Münster
Institute of Biochemistry
Research Group Biochemistry and Structural Biology

Cell polarization is a fundamental morphogenetic process during development and in response to environmental cues. Establishment of cell polarity is coordinated by signaling through small GTPases coupled with polarized membrane trafficking.
The heterodimeric tri-longin domain (TLD) GEF (guanine nucleotide exchange factor) Inturned-Fuzzy (IntuFy) has been reported to activate the Rab7 family GTPase Rab23. Together with its accessory subunit Fritz, IntuFy is part of the planar polarity effector (PPE) complex and an essential component of planar cell polarity in Drosophila melanogaster.
In vertebrates, the corresponding GEF is part of the CPLANE (ciliogenesis and planar polarity effector) complex and additionally required for ciliogenesis. However, the mechanisms for function and regulation of IntuFy activity remain poorly understood. Likewise, the intracellular trafficking pathways controlled by Rab23 are not yet know.
We therefore propose a comprehensive functional and structural investigation of Inturned-Fuzzy-Rab23 to gain insight into how lipid and protein interactions control this Rab/GEF module in distinct functional settings. Understanding the plasticity of GEF complex structure in response to different input signals will elucidate the molecular mechanisms of adaptation in membrane trafficking.
Project-related Publications
Klink B.U., Herrmann E., Antoni C., Langemeyer L., Kiontke S., Gatsogiannis C., Ungermann C., Raunser S., Kümmel D. (2022) Structure of the Mon1-Ccz1 complex reveals molecular basis of membrane binding for Rab7 activation. Proc Natl Acad Sci USA 119, e2121494119.
Fitzian K., Brückner A., Brohée L., Zech R., Antoni C., Kiontke S., Gasper R., Linard Matos A.L., Beel S., Wilhelm S., Gerke V., Ungermann C., Nellist M., Raunser S., Demetriades C., Oeckinghaus A., Kümmel, D. (2021) TSC1 binding to lysosomal PIPs is required for TSC complex translocation and mTORC1 regulation. Mol Cell 81, 2705-2721.
Hansmann P., Brückner A., Kiontke S., Berkenfeld B., Seebohm G., Brouillard P., Vikkula M., Jansen F.E., Nellist M., Oeckinghaus A., Kümmel D. (2020) Structural analysis of the TSC2 GAP domain: mechanistic insight into catalysis and pathogenic mutations. Structure 28, 933-942.
Escobar-Hoyos L.F., Penson A., Kannan R., Cho H ., Pan C-H., Singh R.K., Hobbs G.A., Luo, R, Lecomte N., Babu S., Pan F.C., Alonso-Curbelo D., Morris J.P., Askan G., Grbovic-Huezo O., Ogrodowski P., Bermeo J., Saglimbeni J., Cruz C.D., Ho Y-J., Lawrence S.A., Melchor J.P., Goda G.A., Bai K., Pastore A., Hogg S.J., Raghavan S., Bailey P., Chang D.K., Biankin A., Shroyer K.R., Wolpin B.M., Aguirre A.J., Ventura A., Taylor B., Der C.J., Dominguez D., Kümmel D., Oeckinghaus A., Lowe S., Bradley R. Abdel-Wahab O., Leach S.D. (2020) Altered RNA splicing by mutant p53 activates oncogenic RAS signaling in pancreatic cancer. Cancer Cell 38, 198-211.
Langemeyer L., Borchers A.-C., Herrmann E., Füllbrunn N., Han Y., Perz A., Auffarth K., Kümmel D., Ungermann C. (2020). A conserved and regulated mechanism drives endosomal Rab transition. Elife 9, e56090.
Ungermann C., Kümmel D. (2019) Structure of membrane tethers and their role in fusion. Traffic 20,479-490.
Gao J., Langemeyer L., Kümmel D., Reggiori F., Ungermann C. (2018) Molecular mechanism to target the endosomal Mon1-Ccz1 GEF complex to the pre-autophagosomal structure. Elife 7, e31145.
Langemeyer L., Perz A., Kümmel D., Ungermann C. (2018) A Guanine Nucleotide Exchange Factor (GEF) Limits Rab GTPase Driven Membrane Fusion. J Biol Chem 293, 731-739.
Kiontke S., Langemeyer L., Kuhlee A., Schuback S., Raunser S., Ungermann C., Kümmel D. (2017). Architecture and mechanism of the late endosomal Rab7-like Ypt7 guanine nucleotide exchange factor complex Mon1–Ccz1. Nat Commun 8, 14034.
Zech R., Kiontke S., Mueller U., Oeckinghaus A., Kümmel D. (2016) Structure of the Tuberous Sclerosis Complex 2 (TSC2) N-terminus Provides Insight into Complex Assembly and Tuberous Sclerosis Pathogenesis. J Biol Chem 291, 20008-20020.