Project P9 | Joost Holthuis
Plasticity and biological relevance of organellar lipid codes
We determine how imbalances in organellar lipid codes caused by pathogenic variants of sphingomyelin synthase SMS2 affect secretory pathway function in osteogenic cells to unravel the mechanistic basis of a severe bone disease.
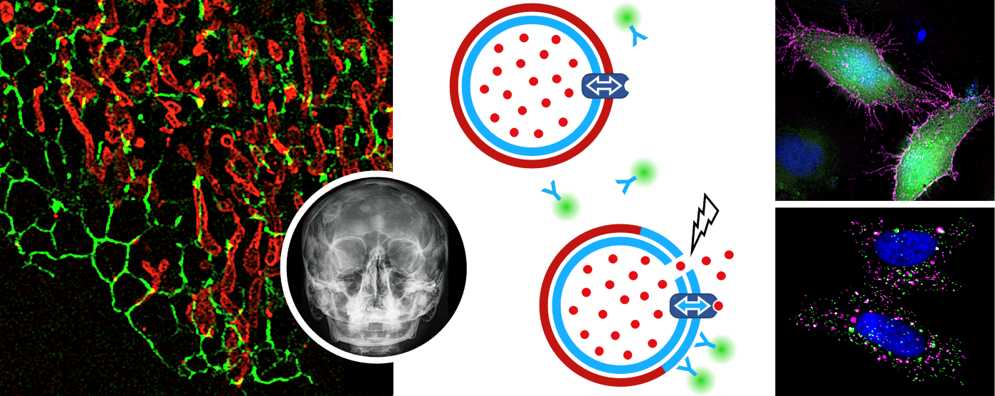
© Joost Holthuis; skull: Pekkinen et al. 2019, license: CC BY 4.0
Project Summary
Prof. Dr. Joost Holthuis
Osnabrück University
School of Biology/Chemistry
Research Group Molecular Cell Biology

The identity and function of cellular organelles critically rely on information encoded in their lipid bilayers. Deciphering these “lipid codes” is a challenging task as they are based on the collective properties of the bulk lipids and because cells exploit a highly interconnected metabolic network to preserve an optimal lipid composition for each organelle. This functional plasticity is critical as perturbations of the lipid code can have profound consequences for a proper functioning of organelles, cells and organisms.
Sphingomyelin (SM) is concentrated in the exoplasmic leaflet of the plasma membrane (PM) and critically influences the subcellular organization of sterols. We previously reported that the PM-resident SM synthase SMS2 is highly expressed in bone and identified mutations that block its export from the ER as the underlying cause of a congenital form of osteoporosis. Moreover, we observed that pathogenic SMS2 variants abolish the SM gradient along the secretory pathway and break SM asymmetry across cellular bilayers. In parallel, we uncovered Ca2+-activated SM scrambling and turnover as a novel mechanism to drive an outward budding of cellular bilayers.
Besides interfering with a timely release of bone critical proteins by undermining the biogenic function of the ER, we envision that pathogenic SMS2 variants may affect the formation of matrix vesicles by osteogenic cells, a process critical for bone mineralization. Here, we seek to unravel the functional implications of, and compensatory cellular responses to disease-induced imbalances in organellar lipid codes, with a main focus on aberrant distributions of the bulk lipid SM.
Project-related Publications
Sokoya, T., Parolek, J., Foged, M.M., Danylchuk, D.I., Bozan, M., Sarkar, B., Hilderink, A., Philippi, M., Botto, L.D., Terhal, P.A., Mäkitie, O., Piehler, J., Kim, Y., Burd, C.G., Klymchenko, A.S., Maeda, K., Holthuis, J.C. (2022). Pathogenic variants of sphingomyelin synthase SMS2 disrupt lipid landscapes in the secretory pathway. Elife. 11: e79278.
Niekamp, P., Scharte, F., Sokoya, T., Vittadello, L., Kim, Y., Deng, Y., Südhoff, E., Hilderink, A., Imlau, M., Clarke, C.J., Hensel, M., Burd, C.G., Holthuis, J.C. (2022). Ca2+-activated sphingomyelin scrambling and turnover mediate ESCRT-independent lysosomal repair. Nature Commun. 13, 1875.
Niekamp, P., Guzman, G., Leier, H.C., Rashidfarrokhi, A., Richina, V., Pott, F., Barisch, C., Holthuis, J.C., Tafesse, F.G. (2021). Sphingomyelin biosynthesis is essential for phagocytic signaling during Mycobacterium tuberculosis cell entry. mBio 12, e03141-20.
Dadsena, S., Bockelmann, S., Mina, J.G., Hassan, D.G., Korneev, S., Razzera, G., Jahn, H., Niekamp, P., Müller, D., Schneider, M., Tafesse, F.G., Marrink, S.J., Melo, M.N., Holthuis, J.C. (2019). Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nature Commun. 10, 1832.
Pekkinen, M., Terhal, P.A., Botto, L.D., Henning, P., Mäkitie, R.E., Roschger, P., Jain,A., Kol, M., Kjellberg, M.A., Paschalis, E.P., van Gassen, K., Murray, M., Bayrak-Toydemir, P., Magnusson, M.K., Jans, J., Kausar, M., Carey, J.C., Somerharju, P., Lerner, U.H., Vesa, O.M., Klaushofer, K., Holthuis, J.C., Mäkitie, O. (2019). Osteoporosis and skeletal dysplasia caused by pathogenic variants in SGMS2. JCI Insight 4, e126180.
Kol, M., Williams, B., Toombs-Ruane, H., Franquelim, H.G., Korneev, S., Schroeer, C., Schwille, P., Trauner, D., Holthuis, J.C., Frank, J.A. (2019). Optical manipulation of sphingolipid biosynthesis using photoswitchable ceramides. eLife 8, e43230.
Bockelmann, S., Mina, J.G.M., Korneev, S., Hassan, D.G., Müller, D., Hilderink, A., Vlieg, H.C., Raijmakers, R., Heck, A.J.R., Haberkant, P., Holthuis, J.C. (2018). A search for ceramide binding proteins using bifunctional lipid analogues yields CERT-related protein StarD7. J Lipid Res 59, 515-530.
Kol, M., Panatala, R., Nordmann, M., Swart, L., van Suijlekom, L., Cabukusta, B., Hilderink, A., Grabietz, T., Mina, J.G.M., Somerharju, P., Korneev, S., Tafesse, F.G., Holthuis, J.C. (2017). Switching head group selectivity in mammalian sphingolipid biosynthesis by active-site-engineering of sphingomyelin synthases. J Lipid Res 58, 962-973.
Holthuis, J.C., Menon, A.K. (2014). Lipid landscapes and pipelines in membrane homeostasis. Nature 510, 48-57.
Haberkant, P., Raijmakers, R., Wildwater, M., Sachsenheimer, T., Brügger, B., Maeda, K., Houweling, M., Gavin, A.C., Schultz, C., van Meer, G., Heck, A.J., Holthuis, J.C. (2013). In vivo profiling and visualization of cellular protein-lipid interactions using bifunctional fatty acids. Angew. Chem. Int. Ed. Engl. 52, 4033-4038.








